NovaSight announced that the FDA has cleared CureSight, a digital therapy device for amblyopia (lazy eye). CureSight is an eye-tracking-based system aimed for improving visual acuity and stereo acuity by training the visual system to use both eyes simultaneously. The ground-breaking clearance was based on visual outcomes data from a multicenter, randomized, controlled trial in which 103 participants aged 4 to <9 were randomized to CureSight or eye patching—the current gold standard-of-care treatment. This was the first-ever pivotal study in which a digital device was shown to be noninferior to patching for amblyopia treatment in children. The study was conducted in six medical centers in Israel.
Randomized Controlled Trial
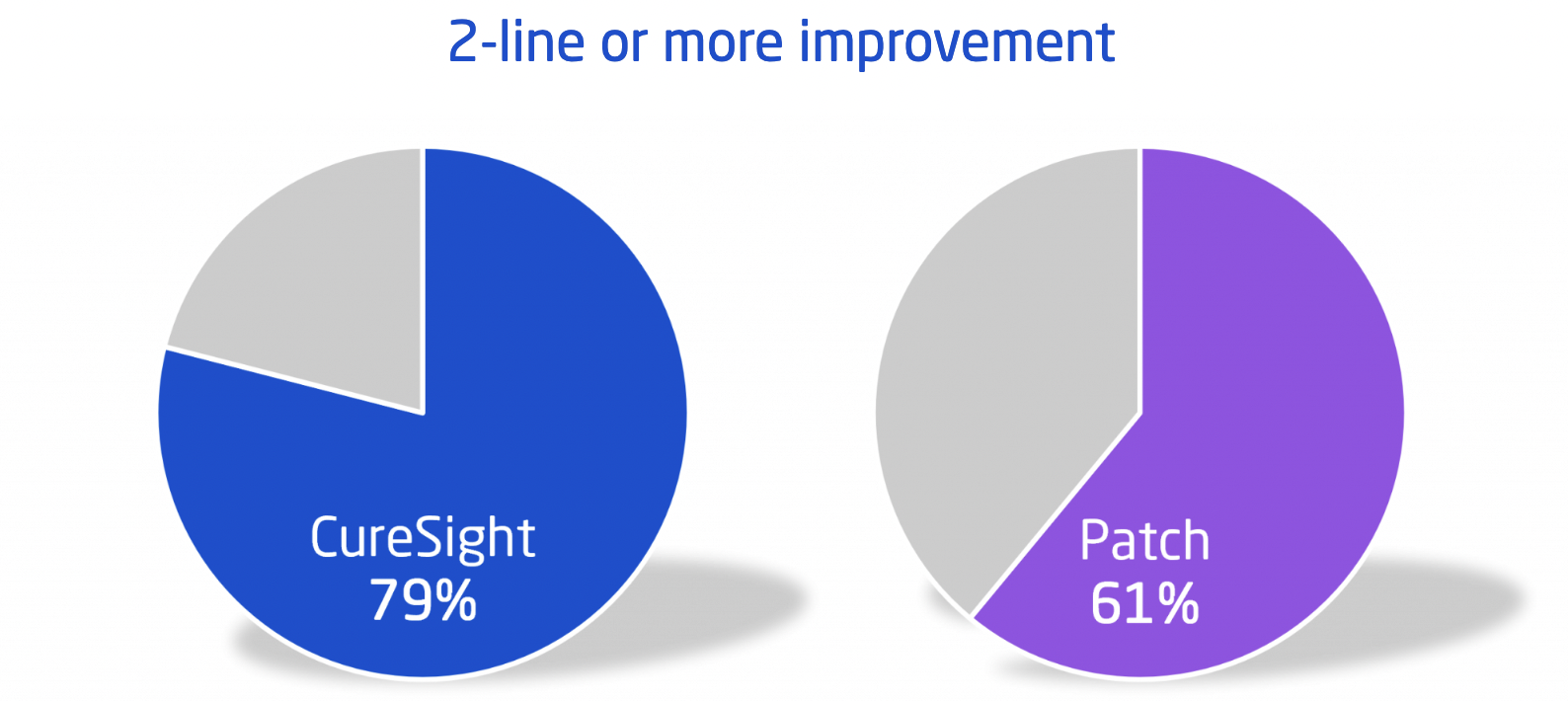
In addition to achieving the noninferiority primary endpoint, the topline analyses from the pivotal study demonstrated that the best corrected visual acuity (BCVA) improvement at week 16 was larger in the treatment group compared to the patching control group. Out of the patients who reached the 16-week visit, 79% in the CureSight group had a BCVA improvement ≥ 2 lines in their amblyopic eye versus 61% of patients in the patching group.
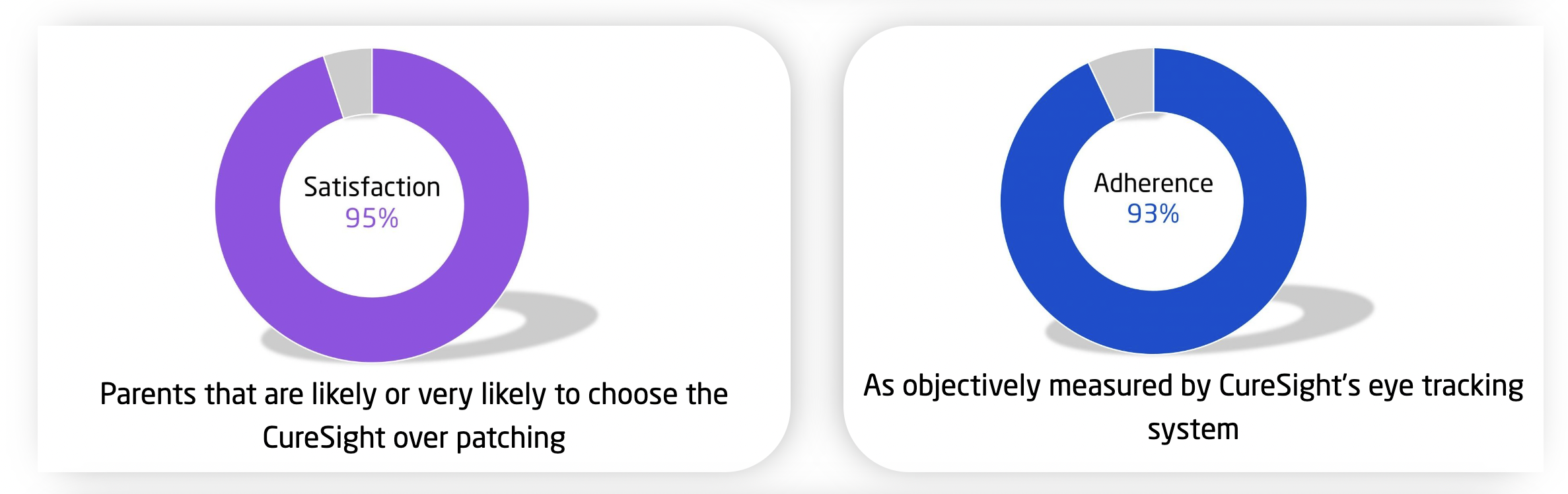
The study also evaluated adherence and patient satisfaction. The mean adherence to CureSight use during the study among subjects evaluated at the week 16 visit (N=43) was 93%. In addition, 95% of parents reported that they are likely or very likely to choose the CureSight digital amblyopia treatment over patching. A significant stereo acuity improvement was observed in both groups. No serious adverse events were observed in either treatment arms–CureSight or patching and all non-serious adverse events were transient and self-limiting.
“Availability of a dichoptic digital treatment of amblyopia that matches the effectiveness of eye patching will help our patients have an alternative that also helps them deal with social and compliance challenges often associated with occlusion therapy. This will help foster a more positive feeling about amblyopia treatment,” said Michael Repka, MD, MBA, a professor of ophthalmology at Johns Hopkins University.
Eye Tracking Technology
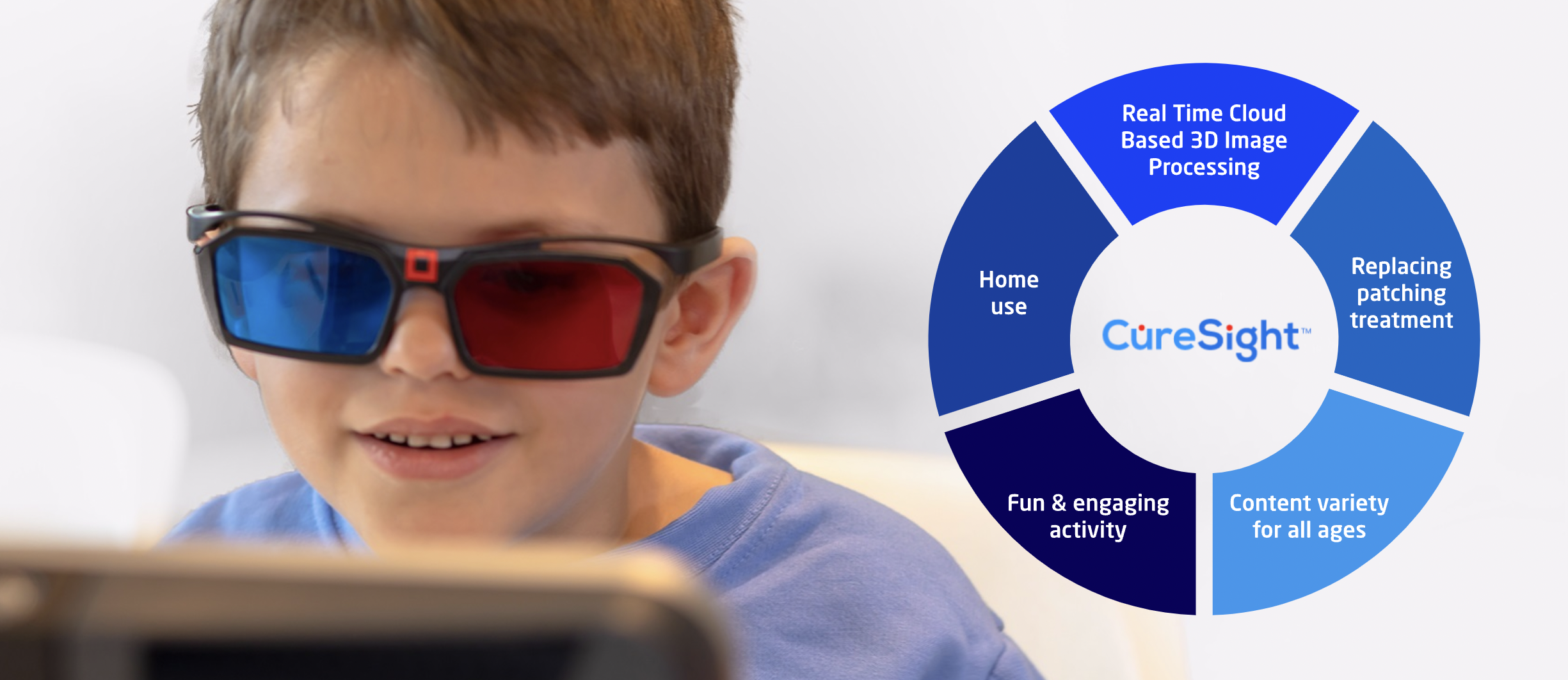
CureSight trains the visual system to use both eyes simultaneously, while the user watches any streamed video content of choice through red-blue treatment glasses. Using sophisticated algorithms and eye-tracking technology, CureSight blurs the center of vision of the image that is shown to the strong eye using real-time image processing according to the momentary eye gaze. This encourages the brain to complete the image’s fine details from the amblyopic eye image and trains the two eyes to work as a team. The device is designed to be used at home, and it shares treatment reports with caregivers via a web portal.
The CureSight web portal allows ECPs to monitor patient treatment progress and compliance. Additionally, a nation-wide Monitoring Center provides comprehensive, beginning-to-end physician and patient support. Directed by practicing ophthalmologists and supported by a team of certified ophthalmic professionals and patient engagement specialists, the Monitoring Center services include compliance monitoring, troubleshooting, billing, and technical support.
“Our study showed that CureSight improves both visual acuity and binocularity in the comfort of the patient’s home, with high adherence and satisfaction. The potential for CureSight to be a standard-of-care-changing technology has increased exponentially with this milestone FDA clearance,” said Ran Yam, NovaSight co-founder and CEO. “The standard of care eye patching treatment, which hasn’t changed for centuries, can be effective if the child adheres to the treatment. However, self-esteem issues and discomfort can limit compliance to be below 50%, and this results in patients suffering from reduced vision in one eye, limited coordination skills, and impaired depth perception in adulthood. We are gratified that FDA clearance together with the 3 unique CPT codes assigned by the American Medical Association (AMA) earlier this year will make access to CureSight possible for patients whose future vision and eye health depend on the treatment that they receive in their early years,” he added
Current Procedural Terminology (CPT) codes are billing codes that allow physicians and service providers the opportunity to potentially be reimbursed by health insurance plans for their services. The assignment of the eye tracking based amblyopia treatment unique CPT codes, for both the home training and physician remote monitoring work, marks an important step toward health insurance reimbursement for CureSight and large availability to patients across the US.
NovaSight recently raised $7 million in the form of a SAFE (Simple Agreement for Future Equity) round bringing the total amount raised by the company to $26 million. The funds are dedicated to targeted commercialization of CureSight in the US, which Mr. Yam notes is already underway.
